

Einstein mathematically expressed the statistical nature of the three possible radiative transition routes (spontaneous emission, stimulated emission, and absorption) with the so-called Einstein coefficients and quantified the relations between the three processes. As with absorption, the probability of stimulated emission is proportional to the intensity of the light bathing the atom. In stimulated emission the presence of photons with an appropriate energy triggers an atom in an excited state to emit a photon of identical energy and to make a transition to a lower state. In 1917 Einstein, though not knowing the exact mechanisms for the emission and absorption of photons, showed through thermodynamic arguments that there must be a third type of radiative transition in an atom-stimulated emission. The absorption of a photon by an atom is also a probabilistic event, with the probability per unit time being proportional to the intensity of the light falling on the atom.
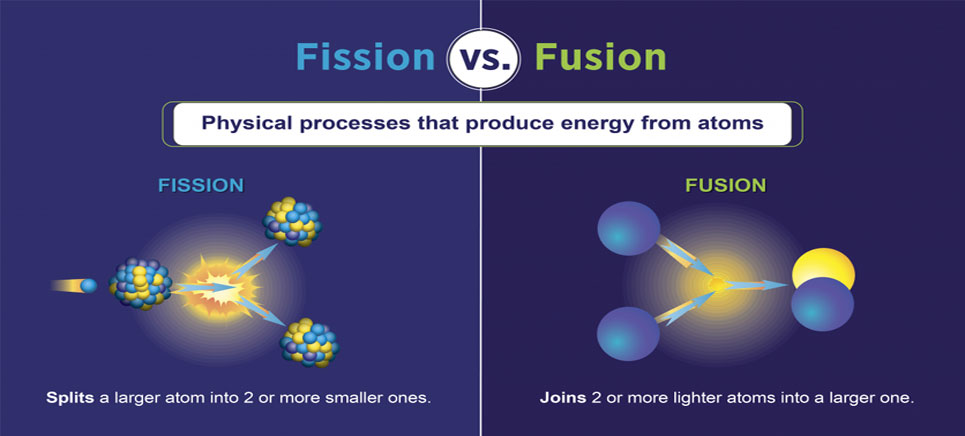
For many excited states of atoms, the average time before the spontaneous emission of a photon is on the order of 10 −9 to 10 −8 second. The emission of a photon is a probabilistic event that is, the likelihood of its occurrence is described by a probability per unit time. This fundamental process is called spontaneous emission. When an isolated atom is excited into a high-energy state, it generally remains in the excited state for a short time before emitting a photon and making a transition to a lower energy state. An electron of relatively high energy may jump to a condition of lower energy, giving off the energy difference as electromagnetic radiation. Modern theory explains the emission of light by matter in terms of electronic energy levels. Though Bohr’s model was superseded by quantum mechanics, it still offers a useful, though simplistic, picture of atomic transitions. Conservation of energy determines the energy of the photon and thus the frequency of the emitted or absorbed light. An atom can absorb or emit one photon when an electron makes a transition from one stationary state, or energy level, to another.
In what is known as the Bohr atomic model, the orbiting electrons in an atom are found in only certain allowed “stationary states” with well-defined energies. Then, in 1913, Danish physicist Niels Bohr proposed a model for the hydrogen atom that succeeded in explaining the regularities of its spectrum. Attempts to describe the origin of the emission and absorption lines (i.e., the frequencies of emission and absorption) of even the simplest atom, hydrogen, in the framework of classical mechanics and electromagnetism failed miserably. The study of the emission and absorption spectra of atoms was crucial to the development of a successful theory of atomic structure. That materials, when heated in flames or put in electrical discharges, emit light at well-defined and characteristic frequencies was known by the mid-19th century.
#Lighttable vs atom how to#
COVID-19 Portal While this global health crisis continues to evolve, it can be useful to look to past pandemics to better understand how to respond today.
Student Portal Britannica is the ultimate student resource for key school subjects like history, government, literature, and more.From tech to household and wellness products. Britannica Explains In these videos, Britannica explains a variety of topics and answers frequently asked questions.This Time in History In these videos, find out what happened this month (or any month!) in history.#WTFact Videos In #WTFact Britannica shares some of the most bizarre facts we can find.Demystified Videos In Demystified, Britannica has all the answers to your burning questions.Britannica Classics Check out these retro videos from Encyclopedia Britannica’s archives.


 0 kommentar(er)
0 kommentar(er)
